The Chemistry of Diamonds and Graphite: A Tale of Carbon Allotropes
Did you know that diamonds and graphite are made up of same element which is Carbon? Even though both was made up of carbon, diamonds are costlier than graphite.
Graphite and diamonds are two of the most well-known forms of carbon, demonstrating the element's extraordinary adaptability. These two types of carbon are made of the same element, but because of their various atomic configurations, they have quite different physical properties. This article focuses into the chemistry of graphite and diamonds, examining the structural variations, bonding configurations, and distinctive properties that set each allotrope apart.
Diamond: The Sparkling Crystal
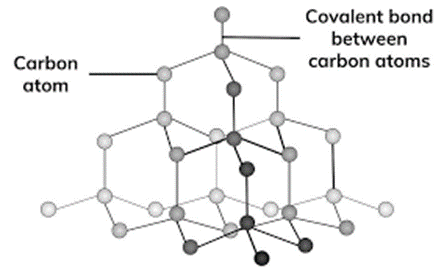
Diamonds are extremely valuable gemstones because of their remarkable hardness and brilliant brilliance. Diamonds are made up of carbon atoms organized in a tetrahedral crystal lattice configuration at the molecular level. A strong and rigid three-dimensional network is formed when each carbon atom makes four strong covalent bonds with its adjacent atoms.
The carbon-carbon bonds in diamonds are sp3 hybridized, meaning that each carbon atom utilizes its three 2p orbitals and one 2s orbital to form four equivalent sp3 hybrid orbitals. These orbitals then overlap with the corresponding orbitals of neighboring carbon atoms, resulting in the formation of sigma (σ) bonds. The strength and stability of the carbon-carbon sigma bonds contribute to the hardness and durability of diamonds.
Graphite: The Slippery Solid
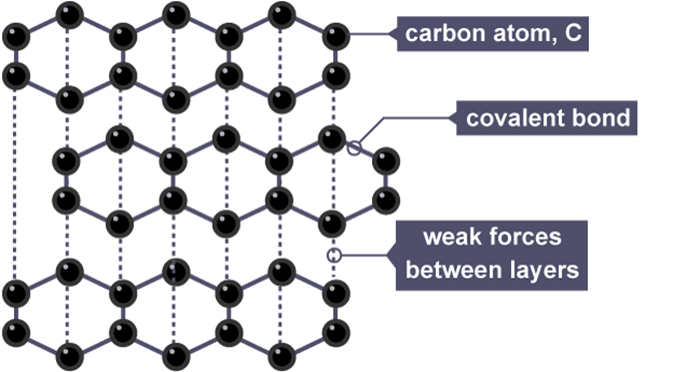
Graphite is a lubricating and slippery substance, in sharp contrast to the hardness of diamonds. Carbon atoms are layered in graphite in a hexagonal pattern, with each atom connected to three others in a trigonal planar arrangement. These layers can easily slip past one another because weak van der Waals forces hold them together.
Graphite has sp2 hybridized carbon-carbon bonds, which means that every carbon atom generates three comparable sp2 hybrid orbitals. While the third orbital produces a pi (π) bond above and below the plane of the carbon atoms, the other two orbitals overlap with the equivalent orbitals of nearby carbon atoms to generate sigma (σ) bonds. Electrical conductivity is made possible by the delocalization of electrons in the graphite layers, which is made possible by the presence of pi bonds.
Contrasting Properties
The atomic configurations and bonding patterns that distinguish diamonds and graphite from one another give rise to their remarkably distinct characteristics. Diamonds are a great material for jewelry since they are translucent, durable, and have a high refractive index. Conversely, graphite serves as the "lead" in pencils and is a solid lubricant in addition to being a good electrical conductor.
In conclusion, the chemistry of diamonds and graphite highlights the diverse nature of carbon allotropes. The unique arrangements of carbon atoms and bonding patterns give rise to distinct physical properties, showcasing the versatility of this element in forming a wide range of materials. From the brilliance of diamonds to the slippery nature of graphite, the study of these carbon allotropes continues to captivate scientists and enthusiasts alike.
Prepared by,
Mohamad Najib Bin Ab Aziz
Chemistry Unit, ASPutra.
Tarikh Input: 18/12/2023 | Kemaskini: 18/12/2023 | hasniah
PERKONGSIAN MEDIA