Water is very important to us for living on this earth. Around 71% of earth is covered by water. We can take a lot of benefits from this creation. Furthermore, water is also used in many chemical reactions.

Water is a molecule that has two hydrogen atoms and one oxygen atom. There are two lone pairs and two bonding pairs around the oxygen atom. Below shows the chemical structure (Lewis structure) of water.
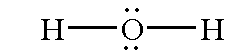 Based on the basic idea of valence shell electron pair repulsion, bond and lone electron pairs in the valence shell of an element repel each other and seek as far apart as possible. The positions assumed by the valence electrons of an atom, define the angles between bonds to surrounding atoms.
Based on the basic idea of valence shell electron pair repulsion, bond and lone electron pairs in the valence shell of an element repel each other and seek as far apart as possible. The positions assumed by the valence electrons of an atom, define the angles between bonds to surrounding atoms.
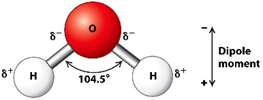
The bond angles determine the geometry of the water molecule. Therefore, the electronic geometry of water is tetrahedral. Meanwhile, the molecular geometry of water is bent. The bond angle of water shows 104.5°.
In terms of the atomic orbital overlapping, the oxygen atom in water molecule undergoes sp3 hybridisation which oriented as tetrahedral shaped as shown below.
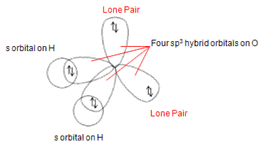
Two hybrid orbitals are overlapped with 1s orbital of hydrogen atom. Another two hybrid orbitals remain unshared because they have fulfilled with two electrons (lone pair).
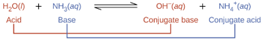
Water has amphoteric properties where it can act as acid when it undergoes reaction with a base, and also can act as base when it’s reacts with an acid. Diagram below shows the example of chemical reaction equation when water acts as an acid when it reacts with ammonia (base molecule).
In terms of the polarity, water is a polar molecule because its direction of the dipole moment cancels each other. Due to that, it behaves as a very good polar solvent where it can dissolve all of the polar solutes such as sodium chloride, NaCl.
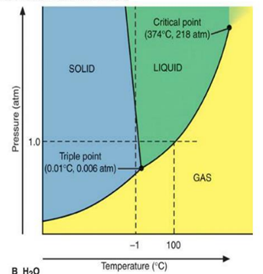
The melting process of water from ice occurs at 0°C (melting point) and may be in vapor formed at 100°C (boiling point). Figure below shows the phase diagram of water.
As a conclusion, although water has a simple structure, but it has its own unique properties. We as human need to appreciate it even it is always regarded a simple creation.
Author : Nur Syafiqah Binti Idris,
Chemistry, ASPer
Tarikh Input: 09/01/2023 | Kemaskini: 09/01/2023 | emma
PERKONGSIAN MEDIA










 Based on the basic idea of valence shell electron pair repulsion, bond and lone electron pairs in the valence shell of an element repel each other and seek as far apart as possible. The positions assumed by the valence electrons of an atom, define the angles between bonds to surrounding atoms.
Based on the basic idea of valence shell electron pair repulsion, bond and lone electron pairs in the valence shell of an element repel each other and seek as far apart as possible. The positions assumed by the valence electrons of an atom, define the angles between bonds to surrounding atoms.


















